
Actions
The ADDIT-CE project is comprised from a bundle of activities designed to achieve the project objectives. The CZ-SK network built within the joint ecosystem will create a mass of concentrated knowledge and know-how to overcome the gaps and tackle the obstacles of R&I development and achieve the objectives by maximising the potential for nucleation and growth of innovative ideas.
The students and junior researchers will be trained to cover state-of-art methodologies of AD diagnostics research through participation in the research project. Their expertise will be elevated by hands-on trainings in methods and techniques employed at the business partners, as well as through lectures by invited renowned experts in the field, which will also offer opportunities for new collaborations.
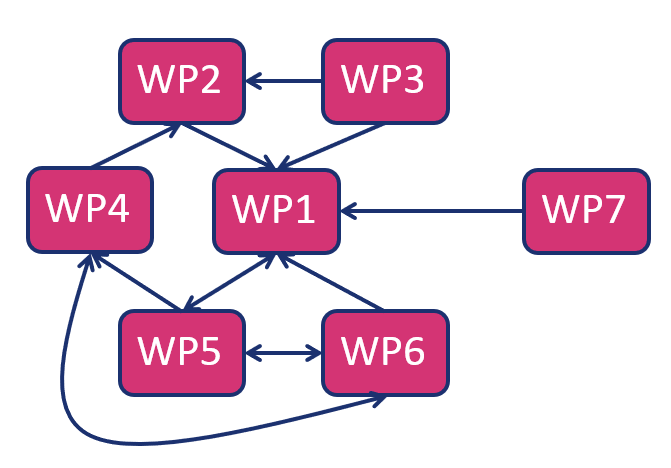
Work packages:
WP1 Development of Joint Cross border R&I Strategy
WP2 Concepts, Designs, and Infrastructure Pre-Plans
WP3 Pathway to Long Term Sustainability
WP4 Development of New Diagnostic Assays for AD Progression
WP5 Engagement of End Users
WP6 Creating A Culture of Innovation
WP7 Project Governance, Management and Ethics
Objectives
1. Creating an excellent knowledge base enabling the development of new AD-diagnostic tools
By sharing our expertise from basic and clinical neuroscience between the two regions and further enhancing it by knowledge exchange networking with top neuroscience experts outside the consortium, we will speed up the development of new diagnostic tools for AD and harmonise the processes of molecular diagnostics across the border.
2. Stimulating interaction between academic and private sector developing AD-diagnostic assays
Through the ADDIT-CE collaborative research project (WP4), proposing further collaborations with industry on brokerage events (WP1), and launching a pilot programme of industrial PhD to bring together academic and industry-based research (WP6), we will contribute to the expected call outcome – strengthened linkages between science and business.
3. Developing common plans for R&I development and infrastructure investments
The successful implementation of ADDIT-CE will result in the mobilisation of regional, national and international
funding as well as private and venture capital. The Joint Strategy will feature pilot R&I projects (WP2) in the field of AD diagnostics aligned with the regional innovation strategies for smart specialisation (RIS3). The rise in excellence, innovation potential and collaboration opportunities will bring about an increase in success rate of attracting competitive funding and private capital (WP3).
4. Joining forces of private and academic research to identify new tau protein, apolipoprotein E (ApoE) and miRNA biomarkers for AD, modernise the standard of AD diagnostics, and validate it for clinical use
We aim to move from the old-fashioned NINCDS-ADRDA diagnostic criteria based solely on purely clinical evaluation to the NIA-AA clinical criteria with biomarker support and/or IWG criteria where clinical and biomarker features must be present for diagnosis. Therefore, we plan to integrate biomarkers from body fluids (blood, CSF) and imaging into diagnostic algorithm. We will focus on new strategies to identify innovative tau, ApoE species and miRNAs to better reflect the course of the disease and increase the chance of early diagnosis.
5. Validating selective biomarkers as efficacy readout for non-pharmacological therapy
The assays and biomarkers developed in WP4 will be used to monitor the efficacy of the non-pharmacology therapy
(such as memory training, mental and social stimulation, and physical exercise programs) on the cohorts recruited in both regions following the international standards (WP5). This will directly involve regional societal actors and end
users (patients and clinical specialists) in ADDIT-CE.
6. Harmonising cohorts of AD patients in Czech and Slovak republics
Currently, there are two independent AD cohorts, one in Memory Centre, Bratislava, and the Czech Brain Aging Study which is a part of the International Clinical Research Centre, St. Anne’s University Hospital in Brno. They use different diagnostic approaches. Thus, we aim to coordinate the processes and the diagnostic tools between both clinical centres to build a fully harmonised Czech-Slovak AD cohort (WP5), aided by the easy understanding between Czech and Slovak languages. We will expand the present databases on the patient cohorts with further state-of-the-art methods and conduct a public information and outreach campaign to recruit patients into our longitudinal observation cohorts. Through these efforts, we will secure a steady stream of high-quality samples for biomarkers validation and elevate the uptake of modern AD diagnostic methods used at the clinical centres.
7. Improve connections between general practitioners, psychiatrists, neurologists, neuropsychologists, healthcare professionals and family caretakers
The aim is to empower general practitioners to take an active role in dementia diagnosis; start dementia diagnosis
pathway already during first patient contact and to connect them to the clinical centres. We will prepare the concept
of a nation-wide epidemiological study on AD to gather the necessary data, implement more efficient patient handling to save resources, and the time of healthcare professionals, patients, and both professional and family caregivers. This activity (WP5, WP6) will be endorsed by governmental and societal partners of ADDIT-CE and we aim to implement the results into the emerging National Plan Against Dementia in Slovakia.


